Author: admin
Completed Initiatives
The BETTER WISE Project

The BETTER WISE project
Although most patients have multiple risks, most guidelines and resources are focused on one specific disease, organ system, or lifestyle risk. Furthermore, cancer survivors and patients living in poverty achieve fewer prevention and screening goals and patients may lack awareness of how lifestyle contributes to cancer and chronic disease. Building on the work of the BETTER trial and the BETTER 2 program, the BETTER WISE (Building on Existing Tools to Improve Cancer and Chronic Disease Prevention and Screening for Wellness of Cancer Survivors and Patients) project will conduct an intervention that includes electronic tools, pathways for cancer survivors, and a tool that screens for poverty.
BETTER WISE is a 6-year project (2016-2022) that brings together diverse stakeholders (policy, practice, research, patients) in Alberta, Ontario, and Newfoundland and Labrador. The primary objective of the BETTER WISE project is to determine if patients aged 40-65, including cancer survivors (breast, colorectal, or prostate) and general health patients (i.e., patients without a previous history of breast, colorectal, or prostate cancer), randomized to receive an individualized visit with a Prevention Practitioner have improved cancer surveillance and general prevention and screening outcomes determined by a composite index as compared to standard care in a wait-list control group twelve months after the initial prevention visit
The BETTER WISE project will comprise of 3 phases:
- Knowledge harmonization and integration – Working with primary care practices and Prevention Practitioners, a revised BETTER WISE tool kit will include blended care pathways for cancer survivors (breast, colorectal, prostate) and cancer and chronic disease prevention and screening (CCDPS), including behavioural lifestyle risk factors and a screen for poverty.
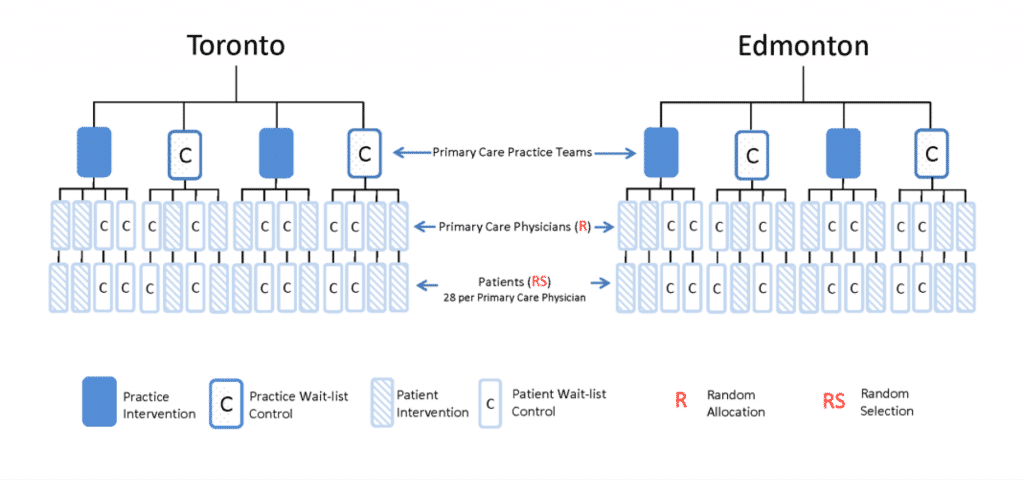
- A pragmatic cluster randomized controlled trial – Sixteen primary care practices, 8 in Alberta, 4 in Ontario, and 4 in Newfoundland and Labrador will participate in the project. It is expected that 2-10 primary care providers (PCPs) from each primary care practice will be engaged, for a total of 64 PCPs across the 3 participating provinces. Patients will be randomized at the physician level to receive an early BETTER WISE intervention or to wait-list control. The BETTER WISE intervention is depicted below.

- Evaluation of the impact of the intervention – The main outcome for the project will be the individual patient-level summary composite index defined as the proportion of CCDPS maneuvers for which the patient was eligible at baseline, that is met (according to pre-defined targets) at 12-month follow-up. Qualitative methods will be used to explore the facilitators and barriers to the implementation and uptake of the BETTER WISE intervention as well as to address any modifications needed to scale and spread the approach and the PP role. An economic assessment will also be undertaken to inform the health care payer and policy makers of the projected cost-benefit impact of investing in the BETTER WISE approach.
For more information about the BETTER WISE project, please contact us.
More information on the Prevention Practitioner role can be found here.
The BETTER WISE project is made possible through a financial contribution from Alberta Innovates – Health Solutions.

Are you a patient participating in the BETTER WISE Project? You can complete your health survey by following the link below:
www.better-survey.caThe BETTER WISE Project Team (2016 – 2022)
Project Lead: Dr. Donna Manca
Project Co-Leads: Dr. Kris Aubrey-Bassler, Dr. Denise Campbell-Scherer, Dr. Eva Grunfeld, Dr. Aisha Lofters, Dr. Melissa Shea-Budgell
Collaborators: A. Bencivenga, G. Bloch, J. Britten, J. Carroll, C. Davis, E. Denga, K. Dong, R. Elford, L. Green, N. Hans, F. Janke, D. Klein, P. Krueger, C. Leduc, R. Lewanczuk, K. McBrien, C. Meaney, R. Moineddin, C. Nykiforuk, M.A. O’Brien, S. Oddie, A. Pinto, M. Rose, S. Ross, G. Salvalaggio, C. Scrimshaw, N. Sopcak, W. Tink, M. Wilson
Community and Policy Partners: C. Campbell, C. Chan, P. Corcoran-Mooney, A. Gogan, R. Hiscock, J. MacWhirter, F. McCrate, B. Meade, K. Milley, R. Goodyear, A. Robinson Vollman, T. Wong
Statistical Analysis: R. Moineddin, C. Meaney.
Economic Assessment: K. McBrien
Project Coordination: C. Fernandes, M. Chow, I. Khalil, K. Sivayoganathan
Research Assistant: D. Ofosu
Students/Trainees: M.K. Blackbyrne, I. Carneiro, M. Kebbe, C. McCartan, F. Nagase, D. Patel, S. Yildirim-Erbasli
Completed Initiatives
BETTER HEALTH: Durham Study

The BETTER HEALTH: Durham study, jointly funded by CIHR and the Canadian Cancer Society, is a study that extends the BETTER Program approach. It consists of a supportive meeting to review chronic disease prevention and screening (CDPS) between a public health nurse taking on the role of Prevention Practitioner and a participant between 40 – 64 years of age who resides in Durham Region of Ontario.
BETTER HEALTH: Durham brings together investigators from Sunnybrook Health Science Centre, St. Michael’s Hospital, Durham Region Health Department and University of Toronto Family & Community Medicine. This project will span from 2017 to 2020.
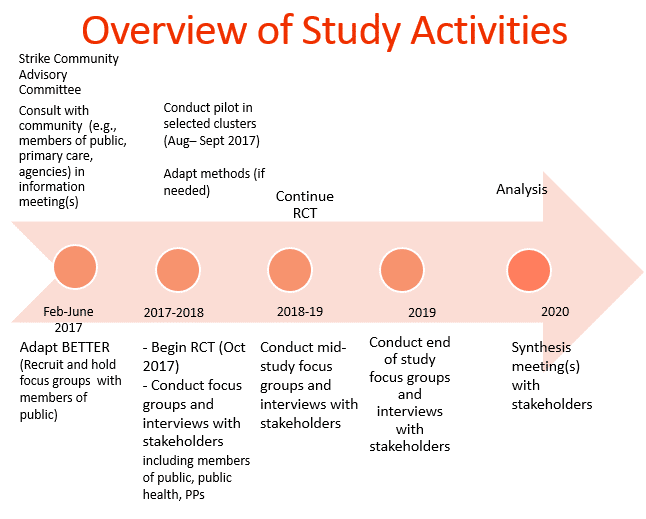
Our goal with BETTER HEALTH: Durham is to adapt the BETTER intervention guided by community-based participatory research (CBPR) principles. Participants are eligible to participate based on the ‘clusters’ where they reside. Clusters are smaller neighborhoods identified as ‘priority neighbourhoods’ by Durham Health Department, and neighbourhoods where cancer screening uptake is known to be low.
Our first phase was the adaptation phase that spanned February to July of 2017. This included conducting focus groups and one-on-one interviews with members of the public and key informants to review preliminary changes of tools, resources and setting. The linked report reviews 5 themes which resulted from this phase.
The second phase, cluster randomization, will aim to have 5 intervention (immediate) and 5 control (wait-list) clusters with 12 participants in each cluster for a total of 120 participants. The intervention arm participants complete a baseline survey by the research coordinator, complete the prevention practitioner visit and then complete their follow up survey 6 months after. For control participants, they are scheduled for their baseline survey, complete their follow up survey in 6 months and then meet with the prevention practitioner. During the visit with the prevention practitioner, the participants are able to set goals, see where their health is at and be connected to other agencies and resources through the Public Health Nurses’ extensive knowledge of community. Participants have been recruited through many means. Some examples of recruitment strategies include: attending local events, newspaper ads, posting recruitment flyers at various approved agencies and communities, Canada Post mail-out, presentations at agencies, social media etc.
The third phase, a qualitative evaluation will take place with enrolled participants, stakeholders and partners throughout the study, who will be asked to answer a range of questions from their experiences to their thoughts of implementations of the study. The qualitative phase will be based on grounded theory principles and informed by the Consolidated Framework for Implementation Research (CFIR).
Through these phases, we plan to share the adapted BETTER knowledge with a wide range of stakeholders, including policy makers, advisors, public health, primary care, community and national organizations, and with the larger research audience, using the Knowledge Transition (KT) plan.
Below compares the original BETTER study in primary care to BETTER HEALTH: Durham.
| BETTER in primary care practices | BETTER HEALTH: DURHAM | |
| Physical location of prevention practitioner (PP) | In primary care team clinics | Embedded in Health Department, Durham Region, for community outreach |
| Identification of participants | From electronic medical record | Community-based recruitment strategies |
| Identification of completed and current behaviours and activities | From electronic medical record, and from self-report in self-administered survey | From self-report responses to survey administered by research coordinator |
| Identification of risk factors | Lab tests, survey, electronic medical record | Self report |
| Motivational interview by PP and goal-setting by participants | By prevention practitioner in primary care team clinics | By prevention practitioners at various community locations |
| Facilitation of goal achievement | Clinic staff, prevention practitioner, links, and self | Prevention practitioners, links, and self |
| Strategy to find primary care physician for participants. | Not applicable | Prevention practitioners supported by primary care strategy engaging physicians and clinics near the clusters. |
| Primary outcome measures | Composite index, expressed as the ratio (multiplied by 100) of the number of eligible CDPS (chronic disease prevention and screening) actions at baseline (denominator) that were subsequently met at follow-up (numerator), measured at the patient level. (G*) | |
| Ascertainment of outcomes | Abstraction from EMR and self-report responses at repeat self-administered survey by prevention practitioner | Self-report responses to survey administered by research assistant |
For more information about the BETTER HEALTH: Durham study, please contact Dr. Lawrence Paszat at LawrencePaszat@sunnybrook.ca